RGUHS Nat. J. Pub. Heal. Sci Vol No: 17 Issue No: 4 pISSN:
Dear Authors,
We invite you to watch this comprehensive video guide on the process of submitting your article online. This video will provide you with step-by-step instructions to ensure a smooth and successful submission.
Thank you for your attention and cooperation.
1Dr. Swati B Shetty, Professor, Department of Periodontics, SDM College of Dental Sciences and Hospital, Dharwad, Karnataka, India.
2Department of Periodontics, SDM College of Dental Sciences and Hospital, Dharwad, Karnataka, India
3SDM Research Institute for Biomedical Sciences, University, Dharwad, Karnataka, India
4SDM Research Institute for Biomedical Sciences, University, Dharwad, Karnataka, India
*Corresponding Author:
Dr. Swati B Shetty, Professor, Department of Periodontics, SDM College of Dental Sciences and Hospital, Dharwad, Karnataka, India., Email: drssetty@gmail.com
Abstract
Background: Primary cultures of gingival and periodontal ligament fibroblasts are an important tool in research and drug development in the field of periodontology
Aim: To put forth a predictable protocol for harvesting primary cultures of gingival and periodontal ligament fibroblasts from healthy and diabetic subjects.
Methods: With prior informed consent, gingival tissue and extracted teeth were collected from 20 donors following aseptic precautions. Samples were washed for two minutes with 1% povidone-iodine before transferring them to tubes containing transport medium supplemented with antibiotic-antimycotic solution. Fibroblasts were harvested from the donor tissue following the explant technique. The complete growth medium used in our study comprised of Dulbecco’s Modified Eagles Medium, 10% foetal bovine serum and 1% antibiotic-antimycotic solution.
Results: The results of this study indicate a higher success rate in healthy subjects (100%) compared with diabetic subjects (70%), and for gingival fibroblasts (90%) compared with periodontal ligament fibroblasts (80%). The main reason for failure of cell culture was yeast contamination. On average, gingival fibroblasts reached 70-80% confluence at 15.6 days in healthy subjects and 16 days in diabetic subjects, whereas periodontal ligament fibroblasts reached confluence at 19.6 days and 21 days, respectively.
Conclusion: The simple protocol described in this article can be used to harvest periodontal and gingival fibroblasts from healthy as well as diabetic subjects with good yield and viability.
Keywords
Downloads
-
1FullTextPDF
Article
Introduction
Wound healing in the periodontium is a complex phenomenon and involves networking between resident cells, growth factors and cytokines. Numerous in vitro models have been used to understand soft tissue healing of periodontal tissues and periodontal regeneration. These in vitro cell culture models allow us to study the species-specific and cell-specific behaviour of cells during wound healing.1 Additionally, in vitro models enable us to understand the cellular processes at the genetic and molecular level. Unlike animal models, these in vitro models are less expensive and present fewer moral and ethical dilemmas.2 In periodontal research, the soft tissue healing responses of the gingiva are evaluated via gingival fibroblasts whereas periodontal ligament fibroblasts have been used to understand the events involved in periodontal regeneration.3
Monolayer cell culture is a widely applied in vitro model which allows for experimentation at the cellular level.4 These monolayer cell cultures are developed from primary cultures or commercially available cell lines. Primary cultures are expanded directly from the host tissues by providing them with optimal conditions in vitro. The cells in the form of primary culture are identical to the host cells physiologically but allow for limited passages as they achieve senescence after certain multiplications.5 Cell lines are easily available, provide an unlimited supply of cells and even bypass ethical concerns. An important point to consider is that cell lines are genetically modified to allow for infinite passages. They also undergo alterations in gene expression due to serial passages and mycoplasma contamination resulting in phenotypic variations.6 Moreover, it has been reported that almost one-third of the cell lines distributed for basic research are misidentified and more than 100 scientific papers have reported contamination with other cell lines.7,8 Though cell lines are useful and offer advantages, they do not fully replicate primary cells. Therefore, it is recommended that the key findings should always be confirmed using primary cells.6
Primary cell culture is not only technique-sensitive but also an expensive area of research. Thus, there is a need for a predictable and reliable technique of primary cell culture to facilitate basic and translational research in periodontology. The goal of our paper was to describe a reproducible protocol for harvesting gingival and periodontal ligament fibroblasts from healthy and diabetic subjects.
Materials and Methods
Materials and Reagents
- Dulbecco's Modified Eagle Medium - High glucose w/4.5 g Glucose per litre, 1 mM Sodium pyruvate, L-Glutamine (Catalogue number - AL007S, HIMEDIA).
- Antibiotic- Antimycotic - w/10,000 U Penicillin, 10 mg Streptomycin and 25 µg Amphotericin B per mL in 0.9% normal saline (Catalogue number - A002, HIMEDIA).
- Phosphate Buffer Saline - Saline 1X w/o Phenol red, Calcium and Magnesium (Catalogue number-TL1006, HIMEDIA).
- Trypsin EDTA - 0.25% Trypsin, 0.038% EDTA in Hanks' Balanced Salt Solution w/o Calcium and Magnesium w/ Phenol red (Catalogue number - TCL048, HIMEDIA).
- Foetal Bovine Serum - (Catalogue number - RM9955, HIMEDIA).
Consumables and Equipment
- Sterile petri dishes
- Sterile tissue forceps
- Sterile scissors
- Sterile B.P handle and Blade no. 11
- Biosafety cabinet (BSL 2, Labcanco)
- CO2 incubator (maintained at 37°C and 5% CO2 )
- Centrifuge (Eppendorf, 5810R)
- Inverted microscope (Nikon, Ts2)
- Automated cell counter (Countess II, Life Technologies)
- Cryostorage tank (-196°C) (LS-3000, Taylor-Wharton)
- Tissue culture plates, cell culture treated (100 mm, 60 mm - Eppendorf)
- Conical centrifuge tubes (15 mL and 50 mL - Eppendorf)
Tissue Harvesting
The study protocol was approved by the institute’s ethical committee before the commencement. Twenty subjects requiring crown lengthening or tooth extraction were recruited in the study with their written consent. The details of the selected subjects have been described in Table 1. The subjects were divided into two main groups - healthy or diabetic depending on their systemic condition. The diabetic patients were recruited if they were on medication for the same and their HbA1c levels were <7 suggesting a well-controlled glycaemic state. The following subjects were excluded from our study:
- Gingival inflammation and/ or clinical attachment loss at the site of interest.
- Smokers
- Pregnancy and lactation
- The presence of any underlying systemic disease in Group I (Healthy) and the presence of systemic diseases other than diabetes mellitus type 2 in Group II (Diabetic)
All patients were advised to perform pre-procedural rinsing with 10 mL of 0.2% chlorhexidine for 45 seconds. Gingival fibroblasts were harvested from gingival tissue of donors undergoing third molar extraction or crown-lengthening procedures (n=10). Periodontal ligament fibroblasts were harvested from atraumatically extracted teeth removed for orthodontic reasons or due to poor restorative prognosis (n=10).
Sample Pre-Processing
All the samples were collected using standard aseptic precautions and washed with 1% povidone-iodine for two minutes, followed by the transport medium. The samples were transferred to a sterile container with the transport medium composed of Dulbecco’s Phosphate Buffered Saline (DPBS) supplemented with 1% antibiotic-antimycotic (10,000 units/mL of Penicillin, 10,000 μg/mL of Streptomycin and Amphotericin B 2.5 mg/L). The sample was stored in the refrigerator till further processing at the cell culture laboratory.
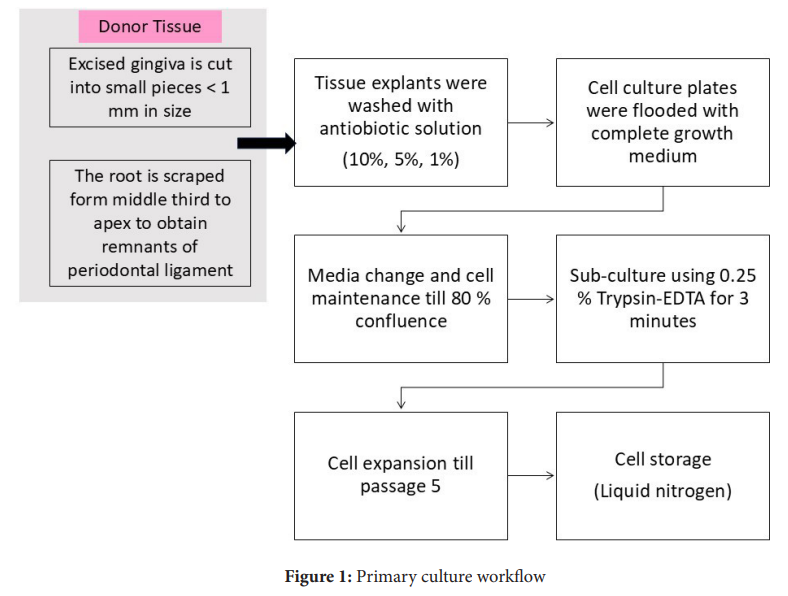
Sample Processing (Figure 1)
At the research centre, the biosafety cabinet was UV sterilized for 30 minutes, prior to the start of the tissue processing. The tissue was transferred into sterile Petri dishes and washed sequentially with 10%, 5%, and finally 1% antibiotic-antimycotic solution. The tissue was then transferred into 100 mm culture plates. The samples with gingival tissue were cut into smaller fragments using sterile scissors or a B.P. blade.9 For harvesting periodontal ligament, the tooth samples were scraped from the middle half to the apex of the root in 60 mm cell culture plates using a sterile 15-number blade.10 Complete growth medium (Dulbecco’s Modified Eagles Medium supplemented with 20% foetal bovine serum and 1% antibiotic and antimycotic solution) was added over the tissue so that the fragments remained in contact with the underlying culture plate surface before the plates were placed in the incubator. The culture plates were observed after 24 hours under the inverted microscope for signs of cell attachment and contamination. The contaminated samples were discarded at this stage. The signs of contamination in samples are turbidity or change of colour of the medium or the appearance of bacterial and / or yeast colonies under the microscope. The culture plates with healthy attached cells were flooded with complete growth medium (Dulbecco’s Modified Eagles Medium supplemented with 20% foetal bovine serum and 1% antibiotic and antimycotic solution) and placed back in the incubator. The culture plates were observed every 2-3 days, the complete growth medium was replaced and the plates were kept back in the incubator.
Cell Propagation and Seeding
The primary cultures were sub-cultured once 70-80% confluence was reached. Before sub-culturing, the complete growth media was pre-warmed in a 37⁰ Celsius water bath and 0.25% Trypsin-EDTA was warmed to room temperature. The culture plate was transferred to the biosafety cabinet. The spent culture medium was aspirated without disturbing the cell monolayer. The cells were washed twice with phosphate buffer saline (PBS) without calcium and magnesium. Two mL of pre-warmed Trypsin-EDTA was added to the culture plate. The culture plate was rocked to ensure complete coverage of the Trypsin-EDTA over the cells and was then transferred back to the incubator. After 2-3 min, the culture plate was observed under the inverted microscope and the dissociation was confirmed (dissociated cells appear as round in contrast to the attached spindle-shaped cells). An equal volume of complete medium was added to the culture plate to neutralize trypsin-EDTA. The contents were transferred to a 15 mL centrifuge tube and the cell suspension was centrifuged at 1000 rpm for six minutes. The supernatant was carefully aspirated and the cell pellet was re-suspended in 1 mL pre-warmed complete growth medium. The total cells, viable and dead cells were counted by Tryphan Blue dye exclusion method using Countess II automated cell counter. The cells were re-seeded in 100 mm cell culture plates with 10 mL of complete growth medium and placed in the CO2 incubator. From this stage, the concentration of FBS was reduced to 10% and growth medium was changed every 2-3 days, following which the cells were observed under the inverted microscope for confluence. The subculturing process was continued till passage 5 (P5) and cells were stored in cryostorage tank at every passage.
Results
The details of the subjects included in the study are described in Table 1 and Table 2. The mean age for successful culture in healthy subjects was 20.6 years (17-28 years), while it was 49.9 years in diabetic subjects (42–54 years). Culture failure was observed in subjects with a mean age of 59 years. Primary cultures of gingival and periodontal ligament fibroblasts could be harvested from all healthy donors, corresponding to a 100% success rate. In diabetic subjects, one gingival sample and two tooth samples were discarded due to contamination, resulting in success rates of 80% and 60%, respectively. Notably, these tissues had been processed more than 12 hours after collection.
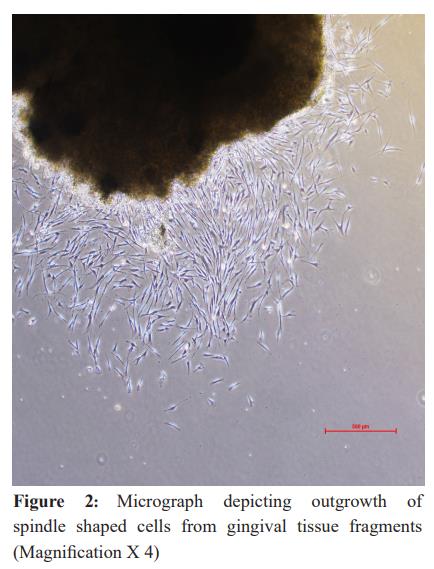
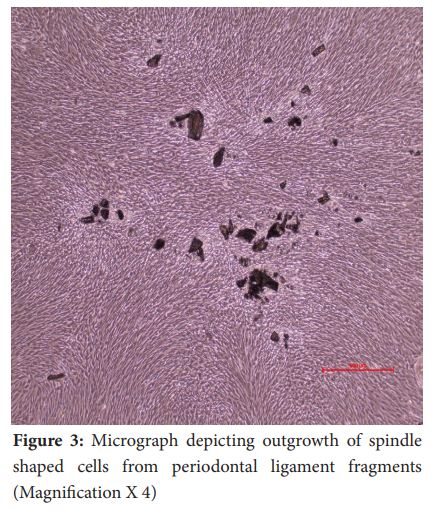
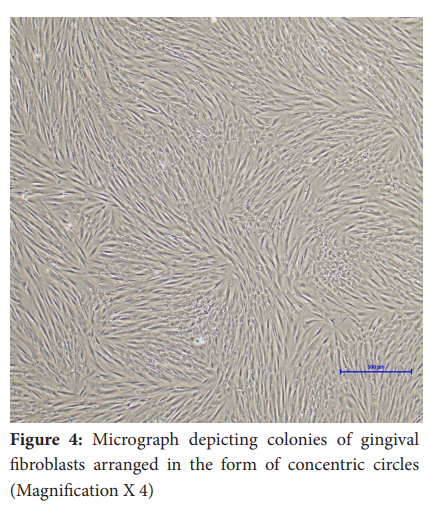
In general, fibroblasts could be observed migrating out of the tissue pieces (tissue out-growth) around day 7 in all the samples (Figure 2 and Figure 3). The attached gingival and periodontal ligament fibroblasts appeared as spindle-shaped cells growing out of the tissue explants. Upon reaching confluence, the culture plates of gingival and periodontal ligament fibroblasts showed peculiar colonies of spindle-shaped cells arranged in concentric circles (Figure 4). On average, gingival fibroblasts reached 70-80% confluence at 15.6 days in healthy subjects and 16 days in diabetic subjects. Periodontal ligament fibroblasts reached confluence at 19.6 days in healthy subjects and 21 days in diabetic subjects.
Discussion
Fibroblasts are known to exhibit the biological and physiological properties that are characteristic of the host and are an indispensable component of basic research.11 Moreover, in recent years, therapeutic modalities are being developed which either deliver fibroblasts to the area of interest or modify the behaviour of resident fibroblasts.12,13 Fibroblast primary culture has therefore become more relevant in both basic and therapeutic research.
Primary culture of fibroblasts can be obtained either by the explant or the enzymatic technique. In the explant method of cell harvesting, host tissue fragments are excised into small pieces measuring < 1 mm and placed into sterile dishes containing the preferred growth medium till cells start migrating and attaching to the culture plate. In the enzymatic method, the tissue samples are partially digested in the presence of proteolytic enzymes such as collagenase or dispase, the resident cells are separated from the host tissue and further cultured in specific growth media. In this study, we employed the explant technique for harvesting fibroblasts as it has numerous advantages. The explant technique allows for reduced tissue processing time, reduced stress on primary cells, and elimination of risk from biological contaminants in enzymes and thus results in a higher cell yield.14,15
The results of our protocol are similar to those of Wanichpakorn et al. who have also suggested the explant method for harvesting gingival and periodontal ligament fibroblasts.16 In addition to the healthy subjects, we established a protocol to predictably harvest periodontal fibroblasts even from Type 2 diabetes mellitus subjects. Previously, the explant technique has been described to harvest dermal fibroblasts from patients with Type 2 diabetes mellitus.17 To the best of our knowledge, this was the first study to propose the protocol for harvesting gingival and periodontal ligament fibroblasts from diabetic subjects.
In this study, yeast contamination was noted to be a greater concern in samples from diabetic subjects, especially those containing extracted teeth. It is interesting to note that gingival tissue obtained from the same patient resulted in a successful primary culture, whereas the sample processed for periodontal ligament cell harvesting showed signs of contamination. Delayed processing (>12 hours) was also a common factor among the samples that showed signs of contamination.
Based on our observations in this study, we would like to propose the crucial steps to prevent contamination in tissue samples -
- aseptic precautions during sample collection
- thorough washing of the collected tissues with 1% povidone-iodine
- ensuring a sterile environment in the cell culture lab
- addition of an antimycotic along with an antibiotic in the transport medium
- minimizing the time between sample collection and processing.
The highlight of our protocol is the application of 1% povidone-iodine after sample collection, which we believe reduced the risk of contamination and culture failure.
Conclusion
Cell culture is a powerful tool in both basic and translational research. Predictable primary culture techniques will become increasingly important as cell therapy, tissue engineering, and 3D bioprinting technologies are the future of periodontal regeneration. The cell culture technique described in this study can be used predictably and successfully to harvest primary cultures of gingival and periodontal ligament fibroblasts from healthy and diabetic subjects.
Conflict of Interest Nil
Supporting File
References
- Weinreb M, Nemcovsky CE. In vitro models for evaluation of periodontal wound healing/ regeneration. Periodontol 2000 2015;68(1):41-54.
- Ud-Din S, Bayat A. Non-animal models of wound healing in cutaneous repair: In silico, in vitro, ex vivo, and in vivo models of wounds and scars in human skin. Wound Repair Regen 2017;25(2): 164-176.
- Lackler KP, Cochran DL, Hoang AM, et al. Development of an in vitro wound healing model for periodontal cells. J Periodontol 2000;71(2): 226-237.
- Honegger P. Overview of cell and tissue culture techniques. Curr Protoc Pharmacol 2001;Chapter 12:Unit12.1.
- Zhao C. Cell culture: in vitro model system and a promising path to in vivo applications. J Histotechnol 2023;46(1):1-4.
- Kaur G, Dufour JM. Cell lines: Valuable tools or useless artifacts. Spermatogenesis 2012;2(1):1-5.
- Bairoch A. The Cellosaurus, a cell-line knowledge resource. J Biomol Tech 2018;29(2):25-38.
- Capes-Davis A, Theodosopoulos G, Atkin I, et al. Check your cultures! A list of cross-contaminated or misidentified cell lines.Int J Cancer 2010;127(1): 1-8.
- Kreisler M, Daubländer M, Willershausen-Zönnchen B, et al. Effect of diode laser irradiation on the survival rate of gingival fibroblast cell cultures. Lasers Surg Med 2001;28(5):445-450.
- Kreisler M, Christoffers AB, Willershausen B, et al. Effect of low-level GaAlAs laser irradiation on the proliferation rate of human periodontal ligament fibroblasts: an in vitro study. J Clin Periodontol 2003;30(4):353-358.
- Jiménez AG, Harper JM. Exploring the role of primary fibroblast cells in comparative physiology: a historical and contemporary overview. Am J Physiol Regul Integr Comp Physiol 2023;325(1):R45-R54.
- des Jardins-Park HE, Foster DS, Longaker MT. Fibroblasts and wound healing: an update. Regen Med 2018;13(5):491-5.
- Bakshi PV, Setty SB, Kulkarni MR. Photobiomodulation of human gingival fibroblasts with diode laser - A systematic review. J Indian Soc Periodontol 2022;26(1):5-12.
- Hendijani F. Explant culture: An advantageous method for isolation of mesenchymal stem cells from human tissues. Cell Prolif 2017;50(2):e12334.
- Priya N, Sarcar S, Majumdar AS, et al. Explant culture: a simple, reproducible, efficient and economic technique for isolation of mesenchymal stromal cells from human adipose tissue and lipoaspirate. J Tissue Eng Regen Med 2014;8(9):706-716.
- Wanichpakorn S, Kedjarune-Laggat U. Primary cell culture from human oral tissue: Gingival keratinocytes, gingival fibroblasts and periodontal ligament fibroblasts. Songklanakarin J Sci Technol 2010;32(4):327-331.
- Rahbar Layegh E, Fadaei Fathabadi F, Lotfinia M, et al. Photobiomodulation therapy improves the growth factor and cytokine secretory profile in human type 2 diabetic fibroblasts. J Photochem Photobiol B 2020;210:111962.